Abstract
Background: CDK8 and its paralog CDK19 are part of the kinase module of the mediator complex, which functions as a bridge between enhancers and core promoters. The CDK8 module functions as a master regulator of transcription and lineage development, including regulation of various oncogenic programs and importantly also hematopoiesis and differentiation. The CDK8/CDK19 inhibitor RVU120 (SEL120) is being investigated in a Phase Ib clinical study (NCT04021368) in AML and HR-MDS patients. Preclinical data indicate the high efficacy of RVU120 in AML models, particularly in cells with stem cell-like characteristics, where the treatment leads to lineage commitment and eventually cell death. Results from the patient cohorts of the dose-escalation phase indicate signs of clinical efficacy, including a complete response (CR) in a relapsed/refractory (R/R) AML patient.
Aim: It is now critical to establish the relationship between preclinical and clinical efficacy results and molecular characteristics to identify actionable biomarkers predicting response to CDK8/CDK19 inhibitors.
Methods: Association between gene mutations and gene expression patterns with responses to RVU120 has been analyzed on 27 genetically annotated AML patient-derived cells (PDCs). The activity and efficacy of RVU120 were assessed in both non-differentiating and differentiating media followed by flow cytometry and bioinformatics analysis. DNMT3A mutant PDCs were implanted intravenously into NSG-SGM3 mice and after disease onset, animals were treated orally with RVU120. Profiling of transcriptional response to RVU120 in DMNT3A mutant cells has been performed by RNA-seq.
The first-in-human Phase1b study CLI120-001 (NCT04021368) of RVU120 is currently enrolling R/R AML and HR-MDS patients, in a dose-escalation design aimed at exploring safety/tolerability and identifying the randomized Phase II dose (RPD2). RVU120 is administered orally, every other day (EOD), 7 total doses per cycle, in 21-day treatment cycles until disease progression/unacceptable toxicity. Local assessment of Bone Marrow (BM) and Peripheral Blood (PB) are performed at different time points according to investigator guidelines to define response to study drug according to Dohner 2017 response criteria.
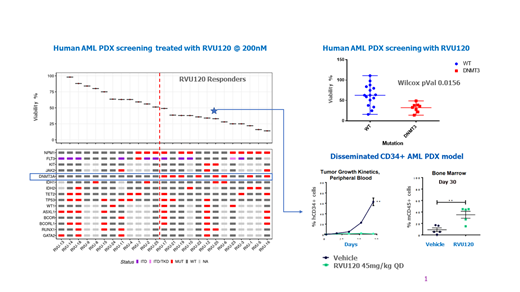
Results: Screening of 27 AML PDCs against RVU120 and other non-related CDK8/CDK19 inhibitors indicated high anti-cancer efficacy in >40% of tested samples (12 out of 27). Correlation of efficacy with the genetic profile of samples showed specific enrichment of DNMT3A mutants (8 out of 12) in the responder group (p=0.015). Notably, efficacy results were further corroborated in vivo in a disseminated PDX AML model, showing complete clearance of blasts positive for DNMT3A mutation and recovery of normal murine BM in animals treated with RVU120. Molecular profiling of responder cells indicated transcriptional reprogramming and lineage commitment.
At the date of this abstract submission, 7 patients have been enrolled into the Phase 1b CLI120-001 trial: 5 AML and 2 HR-MDS patients, median age 73 years, failing 2 median previous lines of therapy. Notably, a 62 YO R/R AML patient that has achieved a CR was positive for DNMT3A R882C mutation. At study entry, this patient had progressed after venetoclax/decitabine, with pancytopenia and >50% BM monocytic blasts and skin leukemia. At the end of the first cycle of the study drug, BM showed complete clearance of blasts with hematological recovery and strong monocytic differentiation starting in cycle 2. Skin leukemia lesions improved gradually during treatment with a complete resolution in cycle 7, resulting in CR. After 1 month from CR patient progressed with 65% BM blasts with the same phenotype as at the study entry.
Conclusion: AML PDCs with DNMT3 mutations show increased sensitivity to RVU120 treatment both in vitro and in vivo. The anti-cancer efficacy of RVU120 was strongly associated with transcriptomic reprogramming and lineage commitment. Preliminary evidence of response to RVU120 has also been shown in a R/R AML patient positive for DNMT3A mutation that has achieved a CR. Further molecular studies in more patients treated with RVU120 are ongoing and could provide evidence for predictive biomarkers of response to RVU120 in AML.
Rzymski: Ryvu Therapeutics: Current Employment, Current equity holder in publicly-traded company. Pakulska: Ryvu Therapeutics: Current Employment, Current equity holder in publicly-traded company. Burris: Boehringer Ingelheim: Consultancy, Other: research grant ; AstraZeneca: Consultancy, Other: research grant ; Bayer: Consultancy, Other: research grant ; Daiichi Sankyo: Consultancy; Grail: Consultancy; Incyte: Consultancy, Other: research support; Novartis: Consultancy, Other: research grant, Expert Testimony; Pfizer: Consultancy, Other: research grant ; Vincerix Pharma: Consultancy; Abbvie: Other: research grant ; Agios: Other: research grant ; ARMO Biosciences: Other: research grant ; Array BioPharma: Other: research grant ; BioAtla: Other: research grant ; BioMed Valley Discoveries: Other: research grant ; Boehringer Ingelheim: Other: research grant ; Bristol Myers Squibb: Other: research grant ; CALGB: Other: research grant ; CicloMed: Other: research grant ; eFFECTOR Therapeutics: Other: research grant ; Lilly: Other: research grant ; EMD Serono: Other: research grant ; Roche/Genetech: Other: research grant ; GlaxoSmithKline: Other: research grant ; Harpoon: Other: research grant ; Hengrui Therapeutics: Other: research grant ; Infinity Pharmaceuticals: Other: research grant ; Janssen: Other: research grant ; Jounce: Other: research grant ; Kymab: Other: research grant ; MacroGenics: Other: research grant ; MedImmune: Other: research grant ; Merck: Other: research grant ; Millennium Pharmaceuticals: Other: research grant ; Moderna: Other: research grant ; Foundation Medicine: Other: research grant ; Revolution Medicine: Other: research grant ; Seattle Genetics: Other: research grant ; Tesaro: Other: research grant ; TG Therapeutics: Other: research grant ; Verastem: Other: research grant ; Vertex Pharmaceuticals: Other: research grant ; XBiotech: Other: research grant ; Zymeworks: Other: research grant ; Arch Oncology: Other: research grant ; Arvinas: Other: research grant ; Coordination Pharmaceuticals: Other: research grant ; NGM Biopharmaceuticals: Other: research grant ; Gossamer Bio: Other: research grant ; Ryvu Therapeutics: Other: research grant ; BioTheryX: Other: research grant ; HCA Healthcare: Other: stock ownership. Obacz: Ryvu Therapeutics: Current Employment, Current equity holder in publicly-traded company. Goller: Ryvu Therapeutics: Current Employment, Current equity holder in publicly-traded company. Combik: Ryvu Therapeutics: Current Employment, Current equity holder in publicly-traded company. Mazan: Ryvu Therapeutics: Current Employment, Current equity holder in publicly-traded company. Juszczynski: Ryvu Therapeutics: Current equity holder in publicly-traded company. Zawadzka: Ryvu Therapeutics: Current Employment, Current equity holder in publicly-traded company. Brzozka: Selvita SA: Current Employment, Current equity holder in publicly-traded company, Membership on an entity's Board of Directors or advisory committees; Ardigen: Current Employment, Membership on an entity's Board of Directors or advisory committees; Ryvu Therapeutics: Current Employment, Current equity holder in publicly-traded company, Membership on an entity's Board of Directors or advisory committees. Shamsili: Ryvu Therapeutics: Current Employment, Current equity holder in publicly-traded company. Angelosanto: Ryvu Therapeutics: Current Employment.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal